Irritable bowel syndrome (IBS) characterized by abdominal pain, altered bowel habits, affects 10-15% of adults in the United States (American College of Gastroenterology, 2025). The gut microbiome plays a crucial role in IBS, influencing both the onset of the condition and the exacerbation of symptoms. Research highlights the significant impact of the microbiome on the intestines and higher-level structures, such as the brain, by producing neurotransmitters and tissue hormones through the microbiome-gut-brain axis.
The central nervous system communicates with various target structures in the intestine, such as the enteric nervous system, intestinal muscles, and intestinal mucosa, primarily through the afferent and efferent fibers of the autonomic nervous system.
This communication influences intestinal motility, immune cell activity, intestinal permeability, and mucus secretion, all of which can alter the diversity and relative proportions of the microbiome.
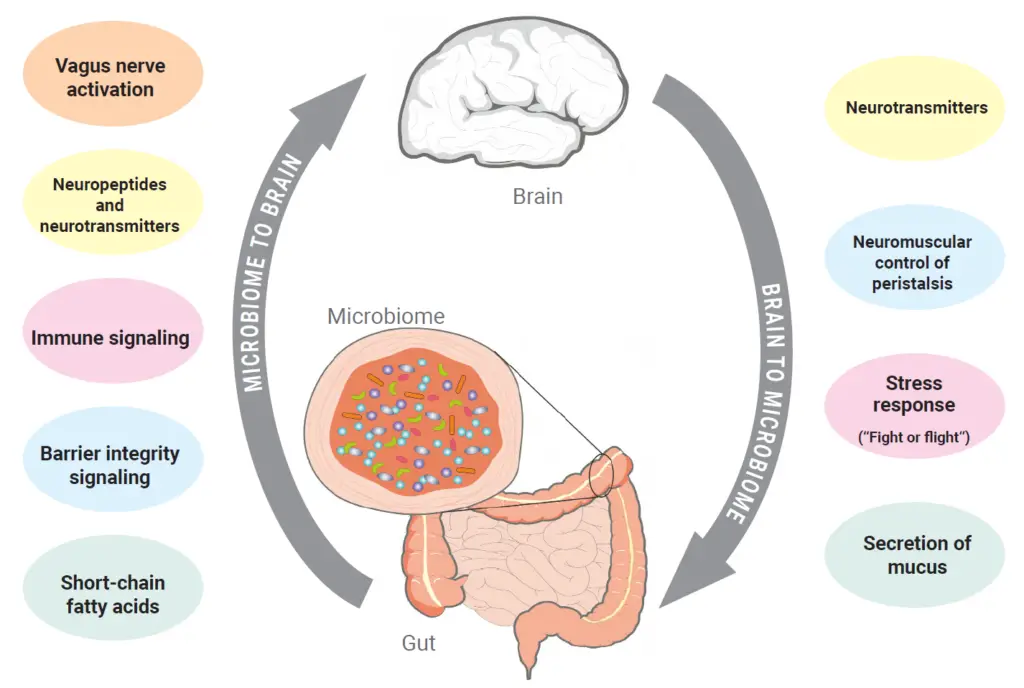
Conversely, the microbiome also influences the intestine and brain through these same factors. In addition to regulating intestinal permeability and interacting with afferent nerve fibers, the microbiome can affect the activity of the enteric nervous system via signaling molecules. The microbiome produces molecules like GABA, serotonin, histamine, and tryptophan, which act locally as neurotransmitters, tissue hormones, and growth factors, thereby activating critical neurological and immunological processes in the intestine (Carabotti et al., 2015).
Neurotransmitters and tissue hormones such as histamine, GABA, serotonin, and its precursor, tryptophan, regulate the production of digestive juices, intestinal motility, and pain perception.
The tissue hormone histamine regulates immunomodulatory processes through mast cells, enterochromaffin cells, and nerve cells. It influences the chemotaxis of eosinophilic and neutrophilic cells, as well as the production of prostaglandins and cytokines (e.g., IL-6 and TNFα).
An imbalance between histamine intake from food or production by intestinal bacteria and its degradation by enzymes such as diamine oxidase (DAO) or histamine- N-methyltransferase (HNMT) can lead to histamine accumulation in the intestine. Elevated histamine levels may cause various disorders, including nausea, diarrhea, headaches, shortness of breath, and tachycardia (Smolinska et al., 2014; Kovacova-Hanuskova et al., 2015).
The neurotransmitter serotonin (5-hydroxytryptamine or 5-HT) plays a crucial role in regulating gut function. Serotonin controls intestinal peristalsis through the 5-HT3 receptor (Horii et al., 2015). Low serotonin levels are associated with constipation (IBS-C) (Dunlop et al., 2005), while very high levels can lead to diarrhea (IBS-D). Additionally, severely elevated serotonin concentrations, combined with overexpression of the 5-HT3 receptor, can result in abdominal pain (Yu et al., 2016).
The amino acid tryptophan is a precursor to serotonin and plays a crucial role in activating repair processes in the intestinal mucosa. Tryptophan stimulates mTOR, leading to increased formation of barrier proteins, defensins, and secretory IgA (Liang et al., 2018). Additionally, tryptophan is a precursor to indole derivatives, which have anti-inflammatory effects (Zelante et al., 2013).
GABA is an inhibiting neurotransmitter in the central and peripheral nervous systems. In IBS, GABA is characterized by its inhibitory effect on visceral pain (Loeza-Alcocer et al., 2019; Icenhour et al., 2019). Accordingly, a low GABA level indicates a high pain sensitivity in IBS (Aggarwal et al., 2018).
Determining the neurotransmitters and tissue hormones produced by bacteria in stool is essential to understand disturbances in the communication between the brain and gut microbiome such as irritable bowel syndrome.

Research suggests that determining the neurotransmitters and tissue hormones produced by bacteria in stool can provide essential insights into disturbances in the communication between the brain and gut microbiome such as irritable bowel syndrome.
Immundiagnostik, Inc. offers a panel of ELISAs to help researchers investigate the microbiome-gut-brain axis in IBS.
All Products Are for Research Use Only. Not for Use in Diagnostic Procedures.
For Laboratory Professional Use Only.
No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.